The world's
first baby has been born using a new "three person" fertility
technique, New Scientist reveals.
The
five-month-old boy has the usual DNA from his mum and dad, plus a tiny bit of
genetic code from a donor.
US doctors took
the unprecedented step to ensure the baby boy would be free of a genetic
condition that his Jordanian mother carries in her genes.
Experts say the
move heralds a new era in medicine and could help other families with rare
genetic conditions.
But they warn
that rigorous checks of this new and controversial technology, called
mitochondrial donation, are needed.
It's not the first time scientists have created babies
that have DNA from three people - that breakthrough began in the late 1990s -
but it is an entirely new and significant method.
Three person babies
Mitochondria are
tiny structures inside nearly every cell of the body that convert food into
usable energy.
Some women carry
genetic defects in mitochondria and they can pass these on to their children.
In the case of
the Jordanian family, it was a disorder called Leigh Syndrome that would have
proved fatal to any baby conceived. The family had already experienced the
heartache of four miscarriages as well as the death of two children - one at
eight months and the other at six years of age.
Leigh syndrome
§ A severe neurological disorder, affecting at least one
in 40,000 new-born babies.
§ Usually becomes apparent during the first year of a
child's life.
§ First signs include vomiting, diarrhoea and difficulty
with swallowing.
§ Causes the progressive loss of movement, and
deterioration of mental functions.
§ Symptoms are linked to the development of patches of
damaged tissue which develop in the brain.
§ Children with the condition usually die within two to
three years, usually because of respiratory failure.
§ Mutations in 75 different genes have been linked to the
condition.
§ Most of those mutations occur in DNA from the nucleus,
but in about one in five cases the culprit is found in mitochondrial DNA.
Scientists have
devised a number of fertility methods to help such families.
The US team, who
travelled to Mexico to carry out the procedure because there are no laws there
that prohibit it, used a method that takes all the vital DNA from the mother's
egg plus healthy mitochondria from a donor egg to create a healthy new egg that
can be fertilised with the father's sperm.
The result is a
baby with 0.1% of their DNA from the donor (mitochondrial DNA) and all the
genetic code for things like hair and eye colour from the mother and father.
Dr John Zhang,
medical director at the New Hope Fertility Centre in New York City, and his
colleagues used the method to make five embryos - only one of them developed
normally.
The UK has
already passed laws to allow the creation of babies from three people.
But the science
does raise ethical questions, including how any child from the technique might
feel about having DNA from three people.
Fertility
experts say it is important to push ahead, but cautiously.
Some have
questioned whether we are only now hearing the success story while failed
attempts could have gone unreported.
Prof Alison
Murdoch, part of the team at Newcastle University that has been at the
forefront of three person IVF work in the UK, said: "The translation of
mitochondrial donation to a clinical procedure is not a race but a goal to be
achieved with caution to ensure both safety and reproducibility."
Critics say the
work is irresponsible.
Dr David King
from the pro-choice group Human Genetics Alert, said: "It is outrageous
that they simply ignored the cautious approach of US regulators and went to
Mexico, because they think they know better. Since when is a simplistic
"to save lives is the ethical thing to do" a balanced medical ethics
approach, especially when no lives were being saved?"
Dr Zhang and his
team say they will answer these questions when they presents their findings at
a meeting of the American Society for Reproductive Medicine in October.
Prof Darren
Griffin, an expert in Genetics at the University of Kent, said: "This
study heralds a new era in preimplantation genetics and represents a novel means
for the treatment of families at risk of transmitting genetic disease.
"With
radical new treatments like this there are always challenging ethical issues,
however any concerns need to be balanced against the ramifications of not
implementing such a technology when families are in need of it."
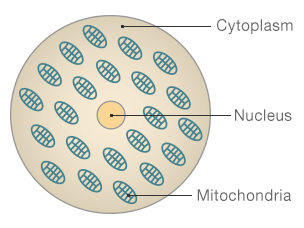
The structure of
a cell
Nucleus: Where
the majority of our DNA is held - this determines how we look and our
personality
Mitochondria:
Often described as the cell's factories, these create the energy to make the
cell function
Cytoplasm: The
jelly like substance that contains the nucleus and mitochondria.